How to Track Pediatric Doses with Apps and Dosing Charts
January 6 2026ANDA Labeling Requirements: What Generic Drug Labels Must Include
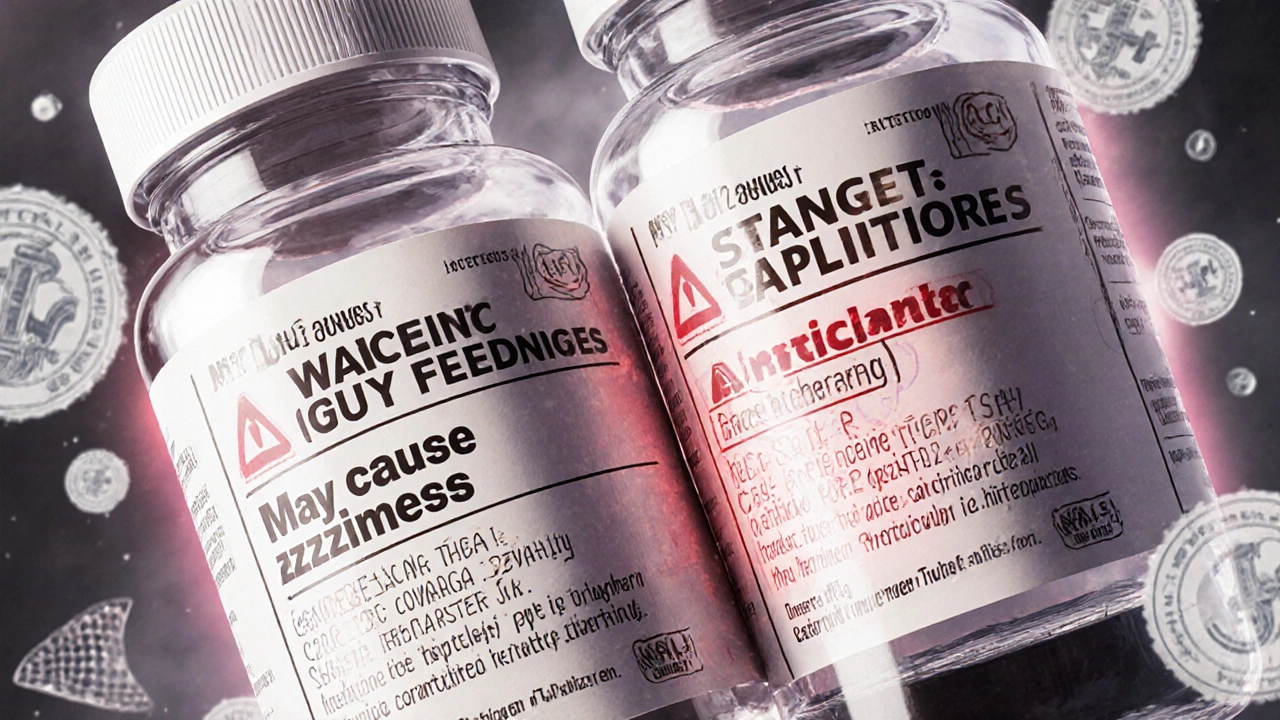
When you pick up a generic pill, the label on the bottle isn’t just a formality—it’s a legal document. The ANDA labeling requirements, the set of rules the FDA enforces for generic drug labels to ensure they match the brand-name version in content, structure, and safety information. Also known as Abbreviated New Drug Application labeling, these rules exist because a generic drug isn’t just a copy of the active ingredient—it must also copy the exact warnings, usage instructions, and risk details. Without strict labeling, patients could miss critical safety info, take the wrong dose, or mix dangerous combinations—all because the label didn’t say what it needed to.
The FDA doesn’t just check if the pill works the same way. They require the generic drug label, the printed or digital information that comes with a generic medication, including boxed warnings, dosing, contraindications, and side effects. Also known as prescribing information, it must mirror the brand-name drug’s label down to the wording. This isn’t about branding—it’s about safety. If the brand-name drug says "do not use if you have kidney disease," the generic must say the same thing, in the same place, with the same emphasis. Even small differences in phrasing can confuse patients or lead to misinterpretation. The FDA labeling, the official guidelines and standards set by the U.S. Food and Drug Administration for how drugs are labeled to ensure consistent, accurate, and safe use. Also known as prescribing information standards, it’s the backbone of this process. Every section—from indications to storage instructions—must align. And if the brand updates its label with new safety data? The generic must update too, within 30 days.
These rules don’t exist in a vacuum. They’re tied directly to bioequivalence—the science that proves a generic drug performs the same in the body. But even if the drug works identically, if the label says something different, it’s not truly equivalent. That’s why the FDA treats labeling as part of the approval process, not an afterthought. You’ll see this connection in posts about narrow therapeutic index drugs, where even tiny labeling errors can lead to overdose or treatment failure. You’ll also see it in discussions about patient concerns with generics—many people worry the pills aren’t as good, but the label is proof they’re held to the same standard.
What does this mean for you? If you’re a patient, you can trust that your generic drug’s label has been reviewed and matched to the brand. If you’re a pharmacist or provider, you know the label you’re handing out is legally identical. And if you’re curious why some generics look different but work the same, the answer starts with the label. Below, you’ll find real-world examples of how these rules play out—whether it’s about avoiding dangerous interactions, understanding side effects, or knowing when a generic is truly safe to use. These aren’t theoretical guidelines. They’re the quiet rules keeping millions of people safe every day.
 17 Nov
17 Nov
Generic Drug Labeling Requirements: What the FDA Actually Mandates
The FDA requires generic drug labels to match brand-name labels exactly, except for manufacturer details. This strict rule ensures consistency but creates dangerous delays in safety updates. Learn what's mandated, why it's risky, and how the system is changing.
Read More...




