Quick Takeaways
- Amiloride is a potassium‑sparing diuretic often used for hypertension and certain kidney problems.
- Human data on amiloride use during pregnancy are limited, but no clear teratogenic signal has emerged.
- Potential risks include low potassium (hypokalemia) for mom and altered fluid balance for the fetus.
- Doctors usually prefer thiazide or loop diuretics when a diuretic is needed in pregnancy, unless amiloride offers a specific advantage.
- Always discuss dosage, monitoring, and alternatives with your obstetrician or maternal‑fetal medicine specialist.
What Is Amiloride?
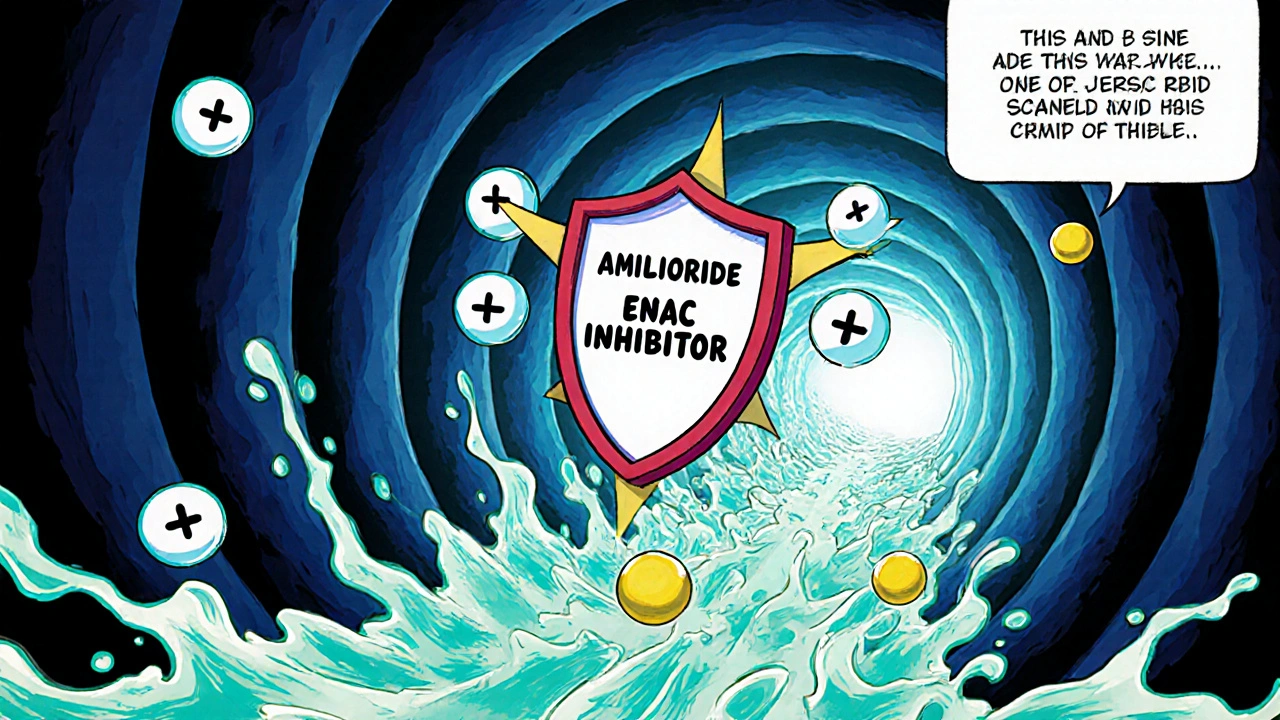
Amiloride is a potassium‑sparing diuretic that works in the distal tubules of the kidney to reduce sodium reabsorption while preserving potassium. It belongs to the class of drugs called potassium‑sparing diuretics and is often prescribed for hypertension, heart‑failure‑related fluid overload, and certain forms of chronic kidney disease (CKD) where potassium loss is a concern.
How Does Amiloride Work?
The drug blocks epithelial sodium channels (ENaC) in the collecting duct. By stopping sodium from entering the cells, water follows, leading to increased urine output. At the same time, because potassium cannot leave the cells as easily, blood potassium levels stay higher compared to loop or thiazide diuretics. This mechanism makes amiloride attractive when clinicians want to avoid hypokalemia.
Use of Amiloride in Pregnancy: What the Evidence Says
When it comes to Amiloride pregnancy safety, the data pool is thin. Most of what we know comes from:
- Animal studies that showed no major birth defects at doses far above human therapeutic levels.
- Case reports and small series of pregnant women who were prescribed amiloride for hypertension or CKD.
- Pharmacovigilance databases (e.g., FDA’s Adverse Event Reporting System) that have recorded a handful of exposure incidents without a clear pattern of fetal harm.
Overall, neither the U.S. Food and Drug Administration (FDA) nor the Australian Therapeutic Goods Administration (TGA) classifies amiloride as a known teratogen. However, because robust, prospective pregnancy registries are lacking, the drug is usually placed in FDA Category C (risk cannot be ruled out) and TGA’s “C” risk category as well.
Risks & Side Effects for Mom and Baby
Even if amiloride doesn’t directly cause birth defects, its pharmacologic effects can create indirect challenges:
- Hypokalemia: While amiloride is potassium‑sparing, over‑correction of fluid balance or concurrent use of other medications (e.g., beta‑agonists) can still lower potassium, which may affect fetal heart rhythm.
- Volume Depletion: Excessive diuresis can reduce placental perfusion, potentially leading to growth restriction.
- Placental Transfer: Limited studies suggest about 20‑30% of the maternal plasma concentration crosses the placenta, but the clinical relevance is unclear.
- Electrolyte Imbalance: Sodium loss can trigger maternal hypertension or orthostatic symptoms, both of which are risky during pregnancy.
Because of these concerns, clinicians often order regular blood tests (serum potassium, sodium, creatinine) every 2-4 weeks if a pregnant patient stays on amiloride.
Comparing Amiloride to Other Diuretics in Pregnancy
Below is a quick snapshot of how amiloride stacks up against the more commonly used thiazide and loop diuretics when a pregnant patient needs fluid control.
| Diuretic | Typical Indication in Pregnancy | FDA/TGA Risk Category | Key Pregnancy‑Specific Concern | Monitoring Required |
|---|---|---|---|---|
| Amiloride | Hypertension or CKD with risk of hypokalemia | Category C / C | Potential hypokalemia, modest placental transfer | Serum K⁺, creatinine, BP every 2‑4 weeks |
| Hydrochlorothiazide (Thiazide) | Gestational hypertension, mild fluid overload | Category B / B | Can cause hyponatremia, slight rise in uric acid | Electrolytes, uric acid, BP each visit |
| Furosemide (Loop) | Severe pulmonary edema, pre‑eclampsia | Category C / C | High risk of volume depletion, ototoxicity at very high doses | Fluid status, electrolytes, renal function weekly |
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) usually recommend thiazides as first‑line because of more extensive safety data. Amiloride is reserved for cases where preserving potassium is critical.
Practical Tips & What to Discuss with Your Doctor
If you or someone you care for is pregnant and already on amiloride, here’s a checklist to bring to the next appointment:
- Current Dose: Write down the exact milligram amount and frequency.
- Other Medications: List any antihypertensives, supplements (especially potassium or magnesium), and over‑the‑counter drugs.
- Symptoms: Note any dizziness, muscle cramps, palpitations, or swelling.
- Lab Results: Bring the latest blood work showing potassium, sodium, creatinine, and blood pressure readings.
- Alternatives: Ask whether a thiazide (e.g., methyldopa) or a low‑dose loop could be safer for your specific situation.
Doctors may decide to:
- Continue amiloride at the lowest effective dose and schedule tighter labs.
- Switch to a thiazide if blood pressure control is adequate without risking potassium loss.
- Taper off the diuretic entirely if fluid balance is stable and hypertension can be managed with other drug classes.
Remember, no medication decision should be made in isolation. The benefits of controlling blood pressure or fluid overload often outweigh the theoretical risks, but the balance is individualized.
Frequently Asked Questions
Is amiloride safe to take during the first trimester?
There is no strong evidence that amiloride causes birth defects in the first trimester, but the drug remains in Category C because human data are sparse. Most clinicians prefer to avoid any non‑essential diuretic during early pregnancy unless the mother’s condition demands it.
Can amiloride cause low potassium in the baby?
Because amiloride is potassium‑sparing, it actually reduces the risk of fetal hypokalemia compared with other diuretics. However, if the mother becomes severely hypokalemic, the fetus could be affected, so regular monitoring is crucial.
Should I stop amiloride as soon as I find out I’m pregnant?
Don’t stop any medication without talking to your obstetrician. They will weigh the risks of uncontrolled hypertension or fluid overload against the limited data on amiloride and may suggest a safe alternative.
How often should blood tests be done while on amiloride during pregnancy?
Most specialists recommend checking serum potassium, sodium, and creatinine every 2-4 weeks, especially during the second trimester when blood volume expands quickly.
Are there any natural alternatives to amiloride for managing fluid retention?
Dietary changes like reduced sodium intake, adequate hydration, and safe exercise can help mild fluid buildup. However, they rarely replace a prescription diuretic when serious hypertension or kidney disease is present.
Bottom line: amiloride isn’t classified as a proven teratogen, but the evidence base is thin enough that doctors treat it cautiously. By staying informed, keeping labs up to date, and maintaining an open dialogue with your care team, you can navigate the risks and benefits with confidence.





christine badilla
October 25, 2025 AT 15:29