Every time you walk on a sidewalk, drive over a bridge, or step into a building, you’re standing on something made possible by calcium carbonate. It’s not just a mineral you take in supplements-it’s the backbone of modern construction. Without calcium carbonate, cement as we know it wouldn’t exist. And without cement, there’s no concrete, no roads, no skyscrapers. This simple compound, found in limestone, chalk, and marble, is the single most important ingredient in making cement. But why? And how does something so common play such a critical role?
What calcium carbonate actually is in cement
Calcium carbonate (CaCO₃) is a chemical compound made of calcium, carbon, and oxygen. In cement production, it’s almost always sourced from limestone, which is quarried in massive quantities around the world. Limestone isn’t just a rock-it’s a raw material that provides the calcium needed to form the key compounds in cement clinker. When heated to about 1,450°C in a kiln, calcium carbonate breaks down into calcium oxide (quicklime) and carbon dioxide. That calcium oxide then reacts with silica, alumina, and iron oxide to form the four main minerals in clinker: tricalcium silicate, dicalcium silicate, tricalcium aluminate, and tetracalcium aluminoferrite. These minerals are what give cement its strength and ability to harden when mixed with water.
Without calcium carbonate, none of those reactions happen. You’d just have a pile of sand and clay with no binding power. That’s why cement plants don’t just use limestone-they rely on it. In fact, limestone typically makes up 70% to 80% of the raw mix in a cement kiln. The rest is usually clay or shale, which supply the other elements. It’s not an option-it’s a requirement.
How calcium carbonate turns into cement
The process starts with mining. A typical cement plant might use 200,000 tons of limestone per year. That limestone is crushed, blended with other materials like silica from sand or fly ash, and fed into a preheater tower. Here, the raw mix is heated gradually to remove moisture and start breaking down the calcium carbonate. Then it enters the rotary kiln-a massive, rotating steel tube lined with refractory bricks. Inside, temperatures hit 1,450°C. At that point, calcium carbonate decomposes: CaCO₃ → CaO + CO₂.
The released carbon dioxide is a major environmental concern, but it’s unavoidable. That’s why cement production accounts for about 8% of global CO₂ emissions-most of it from this chemical reaction, not just burning fuel. The remaining calcium oxide fuses with other oxides to form clinker nodules, about the size of marbles. These nodules are cooled, then ground into a fine powder with a small amount of gypsum. That powder is Portland cement.
Here’s the key point: calcium carbonate isn’t just a filler. It’s the chemical engine. If you replaced it with any other calcium source-like calcium sulfate or calcium hydroxide-you’d get a different product, not cement. The specific ratios of calcium to silica and alumina determine how fast the cement sets, how strong it gets, and how durable it is over time. Calcium carbonate gives you the right kind of calcium, in the right form, at the right time.
What happens if you skip calcium carbonate
Some companies have tried to reduce cement’s carbon footprint by using alternative materials. Fly ash, slag, and calcined clay are now common additives that replace part of the cement. But even in these low-carbon blends, calcium carbonate is still the starting point. You can’t just remove it. You can only reduce the amount of clinker, not the calcium source.
Experiments have been done with pure calcium hydroxide or calcium silicate as substitutes. None worked. They either didn’t harden properly, took too long to set, or cracked under stress. Cement needs the exact chemistry that calcium carbonate provides when heated. There’s no shortcut. Even the most advanced green cements still begin with limestone. Some newer technologies, like carbon capture systems, are being added to cement plants to trap the CO₂ released from calcium carbonate decomposition. But the mineral itself? Still essential.
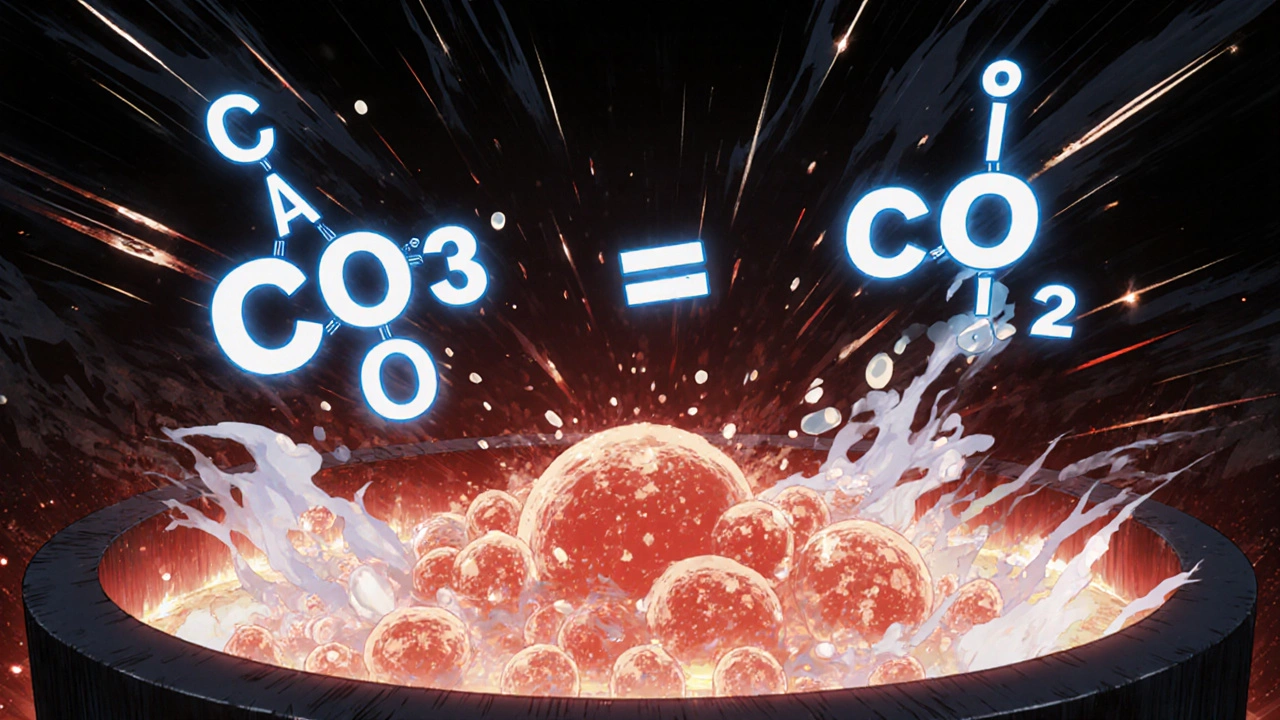
Calcium carbonate and concrete performance
The quality of the limestone directly affects the final product. If the limestone has too much magnesium, it can cause expansion and cracking in hardened concrete. If it’s too pure, the clinker may not form correctly. Cement manufacturers test limestone for its carbonate content, impurities, and particle size before even loading it into the kiln. High-quality limestone has at least 95% calcium carbonate. Lower grades require more blending and careful control.
Concrete made with cement from high-purity limestone tends to have better compressive strength, especially in the first 28 days. That’s critical for construction schedules. In high-rise buildings or bridges, every day of curing matters. Using substandard limestone can delay projects or lead to structural failures later. There’s a reason why major infrastructure projects specify limestone sources by quarry-because not all calcium carbonate is the same.
Global supply and future challenges
China, India, and the United States are the top three producers of cement, and they’re also the top users of limestone. In Australia, where limestone deposits are abundant in Queensland and Western Australia, cement plants like those in Gladstone and Kwinana rely heavily on local quarries. But limestone isn’t infinite. In some regions, high-purity deposits are being depleted. This is pushing manufacturers to look at alternative sources or invest in recycling concrete as a partial replacement.
Even then, recycled concrete still needs new cement to bind it. You can’t make concrete without calcium carbonate. That’s why research is focused on two things: making cement with less limestone and capturing the CO₂ it releases. Carbon capture tech is already being tested at cement plants in Norway and Canada. But the mineral itself? Still non-negotiable.

Why this matters for everyday life
You don’t need to be an engineer to understand why this matters. The roads you drive on, the hospitals you visit, the homes you live in-all depend on cement. And cement depends on calcium carbonate. It’s a silent, invisible foundation. Most people think of concrete as just gray stuff. But behind every slab, every column, every foundation, is a chemical reaction that started with a rock. Calcium carbonate is what turns that rock into something that lasts for decades, even centuries.
It’s also why efforts to reduce cement’s environmental impact are so complex. You can’t just swap out the ingredient. You have to redesign the entire process. That’s why innovations like low-carbon cement blends take years to develop and test. And why, even with all the technology we have, we still can’t build modern cities without it.
Is calcium carbonate the same as limestone?
Limestone is a rock made mostly of calcium carbonate. So yes, in cement production, when we say limestone, we mean calcium carbonate. But not all calcium carbonate comes from limestone-it can also come from chalk or marble. In practice, limestone is the cheapest and most abundant source, so it’s what cement plants use.
Can you make cement without limestone?
No, not with current technology. Limestone provides the calcium needed to form the key compounds in clinker. Without it, the chemical reactions that give cement its strength don’t happen. Some alternative binders exist, like geopolymer cement, but they’re not true cement and aren’t used in most construction. They also still need calcium in some form to match performance.
How much calcium carbonate is in a ton of cement?
About 1.5 to 1.8 tons of limestone (which is roughly 95% calcium carbonate) are needed to produce one ton of cement. That means over 90% of the raw material going into a cement plant is calcium carbonate. The rest is clay or other siliceous materials.
Why does calcium carbonate release CO₂ when heated?
When calcium carbonate is heated above 825°C, it undergoes thermal decomposition: CaCO₃ → CaO + CO₂. The carbon dioxide is released as a gas. This isn’t from burning fuel-it’s a chemical reaction inherent to the mineral. That’s why cement production emits so much CO₂: the process itself creates it, regardless of energy source.
Are there any alternatives to calcium carbonate in cement?
Not yet for mainstream use. Some experimental cements use calcium from industrial waste or synthetic sources, but they’re not widely adopted. Even the most advanced low-carbon cements still rely on limestone as the primary calcium source. Alternatives like fly ash or slag can replace part of the cement, but not the calcium carbonate that starts the process.
What’s next for calcium carbonate in construction
The future of cement isn’t about replacing calcium carbonate-it’s about using it smarter. Carbon capture technology is being integrated into cement plants to trap the CO₂ released during decomposition. Some plants in Europe are already storing it underground or using it to make synthetic aggregates. Others are experimenting with using captured CO₂ to cure concrete, turning waste into strength.
Meanwhile, research into blended cements continues. By adding more supplementary materials like calcined clay, manufacturers can reduce the amount of clinker-and therefore limestone-needed per ton of cement. But even in these mixes, calcium carbonate is still the base. The goal isn’t to eliminate it. It’s to make every kilogram count.
For now, calcium carbonate remains irreplaceable. It’s not glamorous. It doesn’t make headlines. But without it, the modern world would crumble-not just metaphorically, but literally. The roads, the bridges, the buildings-we owe them all to a rock that’s been around for millions of years.





Adarsha Foundation
October 31, 2025 AT 20:50